Apparatus for the study of defect chemistry of solid state
at high temperatures and various activities of gasses, based on the physical method for controlling the partial pressure of different gasses
Determining the equilibrium concentration of defects at high temperatures is essential in studying innovative materials’ physical and chemical properties, e.g., materials for gas sensors, heterogenous catalysis, high-temperature fuel cells, resistive switched memories, piezoelectric/ferroelectric oxides, etc. For such analysis of the type of defect, their concentration and activation energy can be determined using different physical and chemical techniques.
The most popular method of defect chemistry is based on measuring the electrical conductivity as a function of the partial pressure of different oxidizing and reducing gases. Although such investigations are essential and have a long tradition, a crucial point is controlling the gas partial pressure, such as the oxygen partial pressure. The pressure can be adjusted by conventional gas mixing (buffer gases, like CO/CO2 or H2/H2O) or electrochemically using the oxygen pump (YSZ).
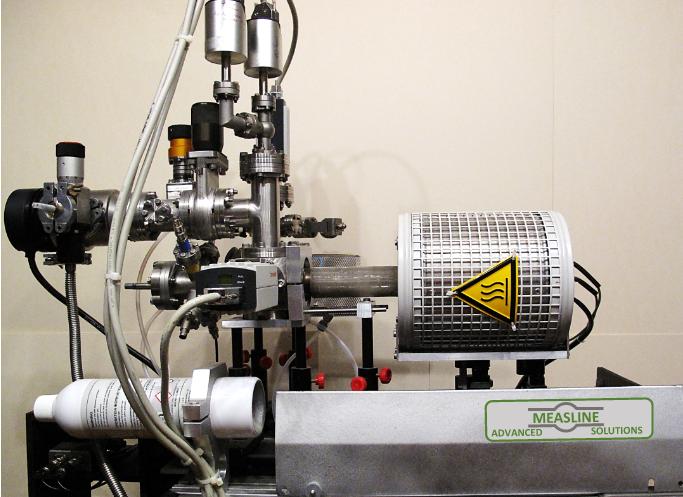
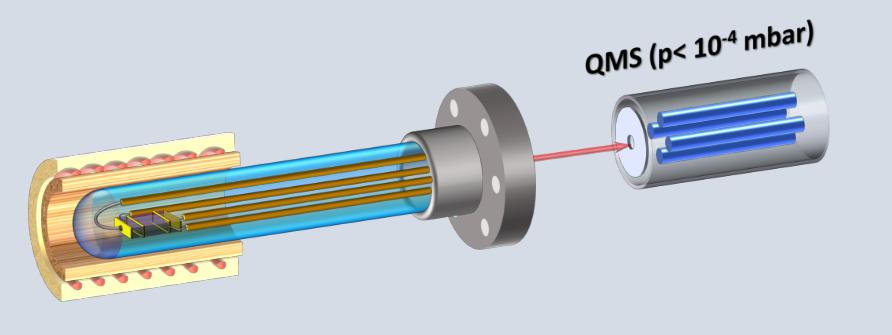
Conductivity measurements under controlled partial pressures for investigating defect chemistry
This new method for controlling partial pressure connected the possibility of generating the XHV or UHV conditions and the precise introduction of the defined doses of various gases in the vacuum system. Additionally, in our apparatus, the partial pressure of the gases is not dependent on the temperature of the mixing gases or the temperature of the oxygen pump, which needs sufficient ionic conductivity; therefore, the YSZ pump or sensor cannot be used below 500 °C.
The advantage of this technique is the ability to include, in the interpretation of data from electrical conductivity measurements (obtained in 4-point geometry), direct information about the physical and chemical properties of the surface, which are essential for the analysis of the mechanism of exchange between the gas and surface interior. This comparison doesn’t directly offer the chemical and electrochemical methods. Using surface-sensitive methods for the study of stoichiometry, electronic structure (using XPS, synchrotron radiation, EELS), crystallography, defect distribution (LEED, STM, AFM), and measurement of local electrical conductivity (with atomic resolution-LCAFM) of the surface layer can broaden the field of analysis while interpreting data from macroscopic conductivity measurements only.
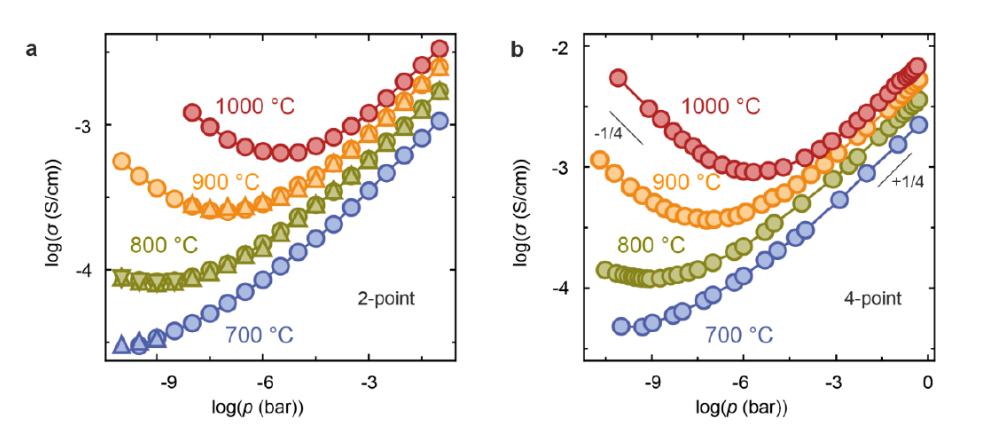
Tests carried out for model oxides (here, the single SrTiO3 crystal) have shown perfect conformity between Brouwer’s diagram and the point defect diagram, which has been determined based on classical control of the partial pressure.
Brouwer diagrams of model materials for defect chemistry (here SrTiO3 single crystal) determined with the apparatus for 2-point and (b) 4-point geometry.
The most significant device features:
- Independent of Gas Temperature and Oxygen Pump Limitations
– Partial pressure control is not dependent on the temperature of gas mixtures or the operation of oxygen pumps
– Eliminates the need for YSZ oxygen pumps, which require ionic conductivity and cannot operate below 500 °C
– Allows experiments at lower temperatures inaccessible to traditional electrochemical control systems - Precise Control of Gas Partial Pressure
– Enables operation under XHV (extremely high vacuum) or UHV (ultra-high vacuum) conditions
– Allows accurate introduction of well-defined doses of various gases into the vacuum system - Possibility to obtain data on Surface Properties via Conductivity Measurements
Enables simultaneous analysis of electrical conductivity (e.g. using 4-point probe geometry) and direct information on surface stoichiometry, electronic structure, defect distribution, local surface conductivity
Specification:
| Method |
DC 4-point method (with triaxial shielding of the electrical connections) |
|
Temperature |
RT-1100°C (±0.05°C ) |
| Basis vacuum |
p<5×10-10 mbar (UHV) p<1×10‾¹² mbar (XHV) |
| Gas Pressure Adjustment |
from 10‾⁹ to 10³mbar – dynamically from the pressure higher than 10‾³–10³ -statically |
|
Determination of gas pressure |
calibrated vacuum gauge optional: the partial pressure of different gases measured using a mass spectrometer
|
|
The resistance of the sample |
should not be higher than 10¹² Ohm |
|
Measurement modes |
I-const. I (100mA- 1fA), Vvar.( ± 200V, adjustable compliance) V-const. Vmax (± 200V), I (100mA- 100fA, adjustable compliance), |
|
Type of samples |
single crystal, ceramic, powder, thin films max. dimension 1×0.5×0.3 cm³ |
|
|
|
|
Method |
AC 4-point methods with lock-in technique ( f =1Hz- 1kHz) |
|
Temperature |
RT-1100°C (±0.05°C) |
|
The resistance of the sample |
should not be higher than 10⁹ Ohm |
We invite you to explore the publication presenting the results achieved with the device described above: Ch. Rodenbucher et al. APL Mater. 9, 011106 (2021)
Explore other system configurations that offer extended functionality and broader application possibilities:
Apparatus for the study of the electro-degradation or resistive switching phenomena
Apparatus for studies of thermally or electrochemically induced desorption processes
System for impedance spectroscopic measurements at high temperature with control of the partial pressure in chamber
We also provide systems for ceramic thin-film deposition as well as cryostats for low-temperature measurements of material properties, including ferroelectric behavior.





