Investigation of desorption and effusion processes based on the measurement of the partial pressure of desorbed gases
In the study of surface physics—including surface defects, electro-degradation phenomena, and controlled desorption—the key factor is the control of gas partial pressure. The pressure can be regulated either through conventional gas mixing (buffer gases such as CO/CO₂ or H₂/H₂O) or electrochemically using an oxygen pump (YSZ). The presented device uses a new method for controlling partial pressure connected the possibility of generating the XHV or UHV conditions and the precise introduction of the defined doses of various gases in the vacuum system. Additionally, in this apparatus, the partial pressure of the gases is not dependent on the temperature of the mixing gases.
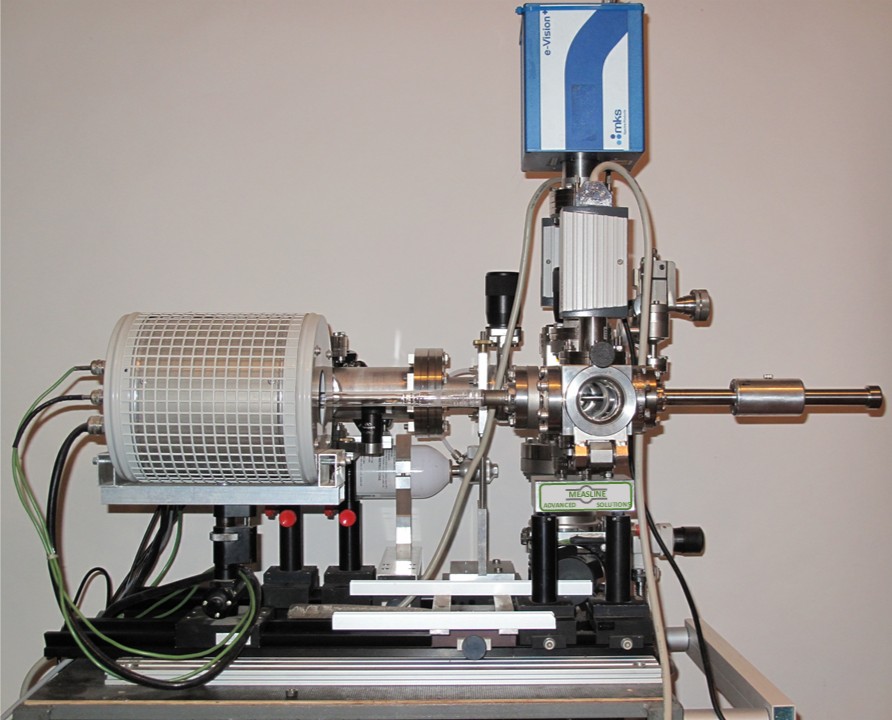
Apparatus for Thermal and Electrochemical Desorption Studies
The system allows the study of the thermal or electrical-induced desorption/effusion phenomena. The information about the desorbed from the surface or effused from bulk species is essential for basic research and technology. Using temperature-programmed or isothermal desorption can be determined essentially for kinetics, statistical thermodynamics, or molecular dynamics parameters for surface or bulk.
The construction of the apparatus allows the separation of the desorption process from the walls of the chambers and the desorption from the sample; namely, the sample can be mechanically introduced into the preheated/baked-out tubes, which are wholly degassed. In addition, the gas loading experiment in the same reaction chamber (for precisely defined temperatures and gas doses) can be obtained and measured using a quadrupole mass spectrometer, which allows subsequently an identification of the gas evolution from the sample induced by heating (isothermal heating or with programmed temperature ramp) or electrochemically.
An integral part of the apparatus is a calibration system, which allows a precise recalculation of the pressure change ( as a function of the time) on the absolute number of atoms or molecules removed from the sample during the desorption
The apparatus allows also for a quick check of the stability of samples, which, by the exposition on vacuum conditions at high temperatures, are in contact with a material that possesses getter properties. The reduction of the local partial pressure, (e.g., oxygen) coming from the getter materials such as Ti, Mo, and Ta ( often applied for the construction of the heating stage or holder) can lead to the complete destruction of the investigated oxides. Using the QMS measurement of the thermally induced effusion from the crucible in which the sample is in contact with getter materials, the range of the stability of the oxides can be determined.
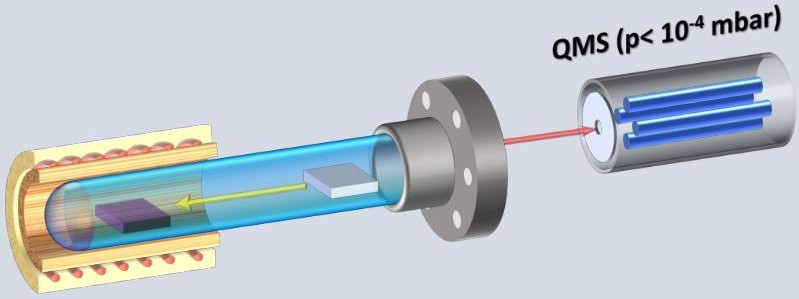
Principle of the measurement of the evolution of gasses during desorption experiments
The apparatus has demonstrated its usefulness, e.g., for the measurement of the evolution of the H₂ from passivated Si for the reduction of different model oxides ( SrTiO3 Figure 5 or BaTiO₃) for electro-degradation (resistive switching Figure 6) of memristive materials or thermal decomposition of oxides in the presence of getters.
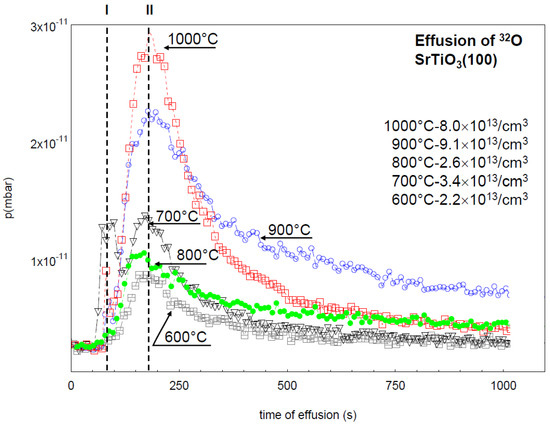
Measurement of the thermo-stimulated desorption of oxygen (obtained using mass-spectrometry) during successive reduction of SrTiO₃ crystal under UHV conditions (T = 600–1000°C). (Szot et al. Crystals, 2018,8, 241; )
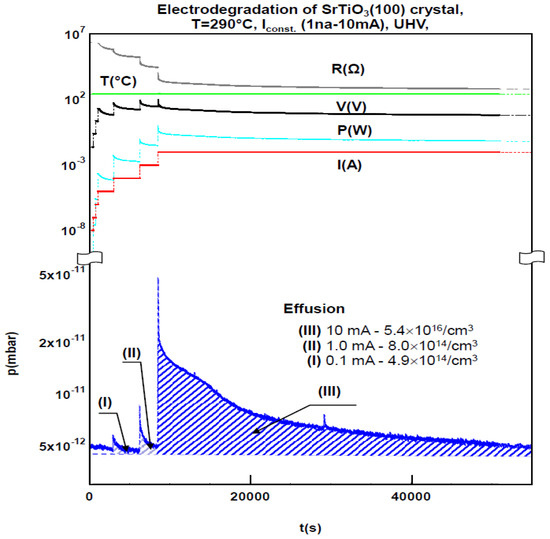
Example of the studies of the effusion processes of oxygen which accompanied the of electro-degradation of SrTiO₃ crystal. (Szot et al., Crystals, 2018,8, 241)
The most significant device features:
-
Analysis of thermally or electrically induced desorption/effusion:
Enables advanced studies of gas release from surfaces and bulk under controlled thermal or electrical conditions. -
Support for temperature-programmed and isothermal desorption:
Suitable for determining kinetic parameters, statistical thermodynamics, and molecular dynamics at the surface or in the bulk material. -
Chamber design ensuring separation of desorption sources:
Allows differentiation between gases desorbed from the sample and those released from the chamber walls. Samples are introduced into preheated and fully degassed tubes. -
Gas dosing and reaction capability:
Enables precise introduction of defined gas doses at controlled temperatures within the same chamber, ideal for reaction studies. -
Quadrupole mass spectrometer (QMS) integration:
Real-time detection and identification of gases released due to thermal ramping or electrochemical stimulation. -
Built-in calibration system:
Allows accurate conversion of pressure changes over time into the absolute number of atoms or molecules desorbed from the sample. -
Assessment of material stability under vacuum:
Facilitates quick verification of the thermal stability of samples exposed to high vacuum and getter materials (e.g., Ti, Mo, Ta). -
Evaluation of getter-induced decomposition:
QMS analysis of effusion from crucibles in contact with getter materials enables determination of oxide degradation thresholds. - Independent of Gas Temperature and Oxygen Pump Limitations
- – Partial pressure control is not dependent on the temperature of gas mixtures or the operation of oxygen pumps
– Eliminates the need for YSZ oxygen pumps, which require ionic conductivity and cannot operate below 500 °C
– Allows experiments at lower temperatures inaccessible to traditional electrochemical control systems - Precise Control of Gas Partial Pressure
– Enables operation under XHV (extremely high vacuum) or UHV (ultra-high vacuum) conditions
– Allows accurate introduction of well-defined doses of various gases into the vacuum system
Specification:
| Vacuum | UHV |
| Detection system | QMS – quadrupole mass spectrometer |
|
Furnace |
100-1400°C |
|
Temperature conditions |
isothermal conditions programmed increasing of the temperature |
|
Kind of sample/ getter |
powder/ powder, ceramics/ powder, solid/ solid |
We invite you to explore the publications presenting the results achieved with the device described above:
Szot et al. Nanomaterials 2024, 14(23), 1944
Rodenbucher et al. Phys. Status Solidi RRL 2017, 11, 1700222
Explore other system configurations that offer extended functionality and broader application possibilities:
Apparatus for the study of defect chemistry of solid state
Apparatus for the study of the electro-degradation or resistive switching phenomena
System for impedance spectroscopic measurements at high temperature with control of the partial pressure in chamber
We also provide systems for ceramic thin-film deposition as well as cryostats for low-temperature measurements of material properties, including ferroelectric behavior.





